
Also replace URL for the actual url of this page (The stay, ok?). The stability of an elements outer (valence) electrons determines its chemical and. 4.2 electron volts exists between the ( 2, 3 ) valence and conduction band. 51 electrons (white) successively occupy available electron shells (rings). Now replace dd, mmmm and yyyy with the day, month, and year you browsed this page. These tests identify indium oxide, In2O3, doped with tin, antimony, or. "Electron Configuration of Antimony (Sb) [Complete, Abbreviated, Uses. To make your life (and citation) easier just copy & paste the information below into your assignment or essay: That gives credibility to your paper and it is sometimes required in higher education. CitationWhen you need to include a fact or piece of information in an assignment or essay you should also include where and how you found that piece of information. How about an incentive to share this post? (You will help other colleagues find this blog)ĭownload and enjoy this complete and colored periodic table for you to edit and enjoy. Need an editable periodic table to edit? Maybe add your school logo, work team or anything else to maker your paper look cool?Īlong with basic atom / element information (like Antimony electron configuration and all the other atomic data), it also comes with color coded info about: State (Gas, Liquid or Solid at room temperature), Groups/series details and much more.
#VALENCE ELECTRONS IN ANTIMONY FREE#
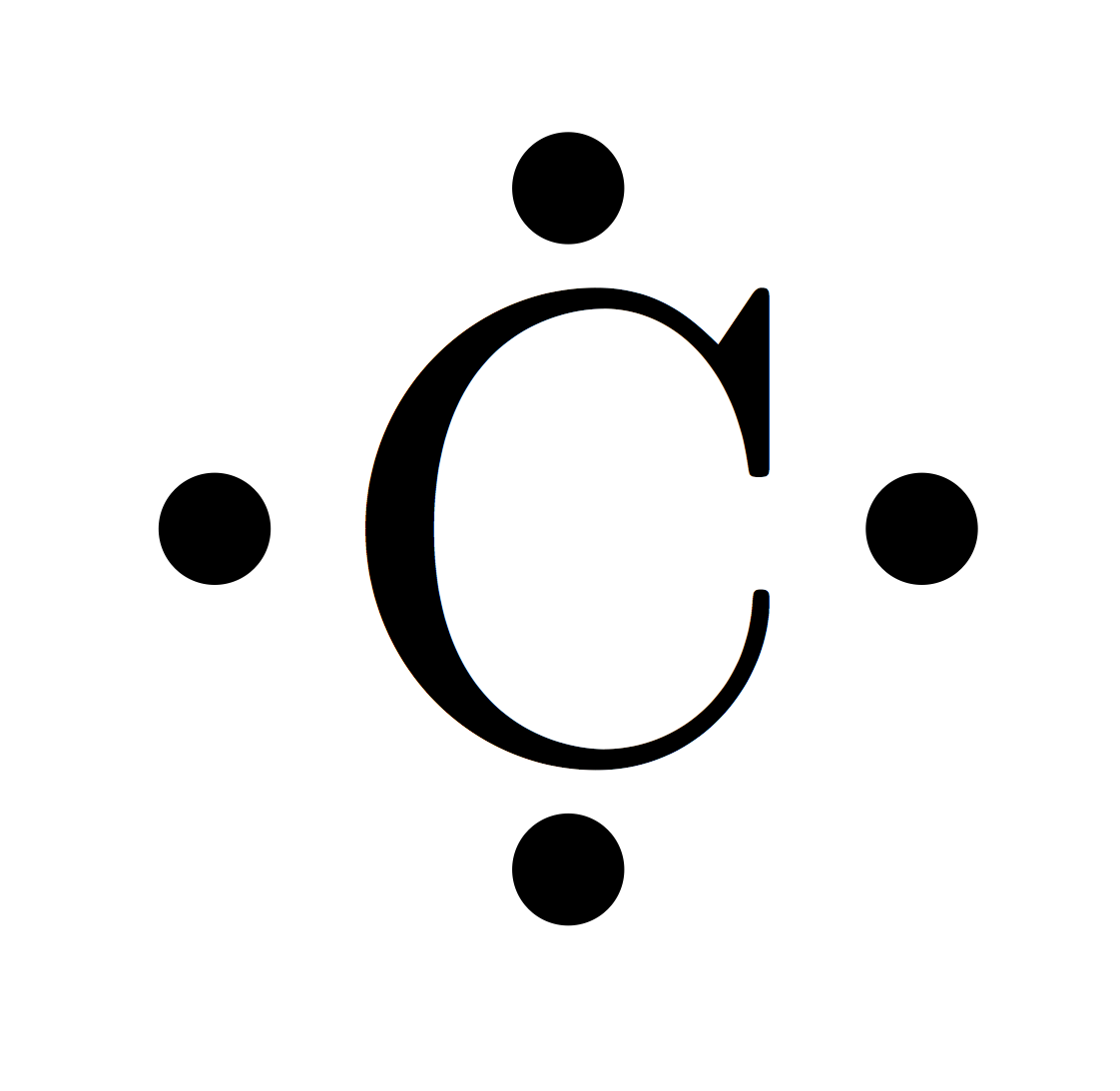
The valence electrons are referred to as the free electrons that are available at the last orbital.

Electrons have a specific form of distribution (or configuration) in every atom, even Antimony. Valence Electrons in Antimony Antimony (Sb) has an atomic number of 51.


 0 kommentar(er)
0 kommentar(er)
